By: Erin Weller
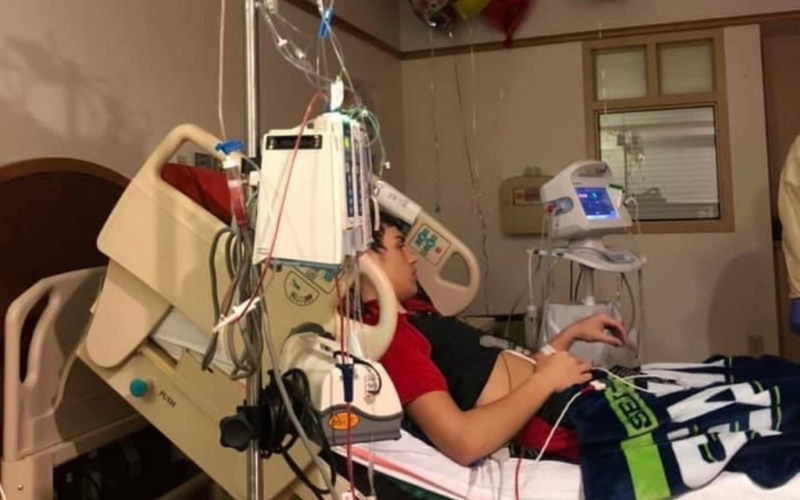
At 14 years old, Austin Ochenshirt was excited to welcome his second little brother into the family. But while they were at the hospital, he could tell something was wrong. What started as a swollen elbow from football camp and a supposed “bone bruise” on his head soon turned into a life-changing discovery.
A week later, Austin was back at the hospital, where testing revealed he had acute myelogenous leukemia (AML).
“It was the worst day of my life,” Austin says.
From there on out he chose to have a positive mindset by looking at cancer as just another bump in the road. “I was always smiling and trying to make everybody else positive,” he explained.
After four rounds of chemotherapy – each keeping him in the hospital for about a month at a time – his family celebrated his victory by throwing a remission party.
Unfortunately, eight months later, Austin relapsed. Still, his hopeful mindset never wavered, and neither did the support of his family nor the efforts from his medical team. This time around, Austin needed a new form of treatment.
At Children’s Hospital of Pittsburgh nearby, the first phase of a clinical trial for kids who had relapsed leukemia had just opened. They were one of several sites running the trial, for a drug called “CPX 351,” across the country.
Dr. Jean Tersak had treated Austin for years at Children’s Hospital of Pittsburgh and believed this new trial drug had the potential of bringing Austin into remission before undergoing a bone marrow transplant. If it worked, the trial drug would lower the chances of yet another relapse while also protecting against potential heart complications in the future.
Austin would be one of the first patients to try it.
He didn’t know what to expect, but his family members were prepared to support each other every step of the way. Whether Austin was playing with his brothers at the hospital to keep them from worrying, or his grandma was taking on the role of number one supporter on days that his mom needed to stay home with Austin’s brother, they stuck it out together.
The trial was a success, and for the second time in his life, Austin was completely overwhelmed with emotion. All he could think was, “I’m free.”
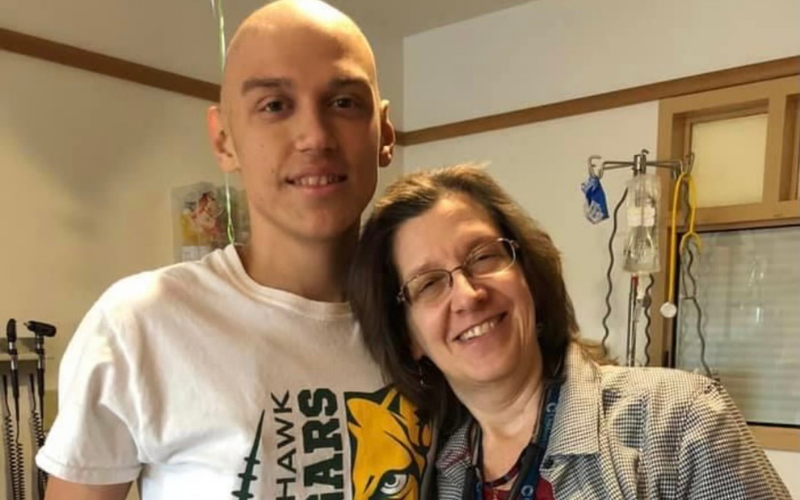
Today, Austin is 19, playing basketball, attending community college, and studying to become a registered nurse, all while cancer-free.
“I’ve been super active, and I feel back to normal since before I was even diagnosed… I’m back to my everyday lifestyle. It’s incredible,” said Austin.
It takes a massive amount of time, energy, effort and, of course, funding to develop new drugs like the one Dr. Tersak used for Austin. But clinical trials also require detailed reporting, constant coordinating, plus rigorous involvement from nurses and physician investigators. Dr. Tersak said, “the funds that are coming in for these trials, they don’t even come close to covering all of the needs to conduct them.”
But with additional funding from Alex’s Lemonade Stand Foundation (ALSF), Children’s Hospital of Pittsburgh was able to afford the necessary infrastructure and staffing to implement their plans.
“If we didn’t have the philanthropic support, we would not be able to open as many trials because we just wouldn’t have the funding to support the staff we need to conduct it in a safe manner,” said Dr. Tersak.
Currently, the CPX 351 trial has made its way to phase three, the final phase before it can reach FDA approval. It’s taken several years, but ALSF support helped make it possible for more clinical trials to be available for kids just like Austin.
“The support that ALSF gave to help us to do the phase one and two portions of the study is now helping patients all over the United States and Canada,” said Dr. Tersak.